Projects
We are working to understand the immune system’s biochemical and genetic profile and to translate our findings into therapies that turn patients’ immune systems against their cancer.
One of the main research areas in the lab is the development of in situ vaccination—a vaccine that is created at the tumor site in cancer patients—with the goal of activating a specific anti-tumor immune response that can target the primary tumor site, and also other cancer cells that have metastasized throughout the body. In a comprehensive study (Nat Med, 2019), we show that combining Flt3L, local tumor irradiation, and the adjuvant poly-ICLC results in the recruitment of dendritic cells that take up tumor antigen and cross-prime T cells to generate systemic anti-tumor immunity, in a murine lymphoma model and in lymphoma patients in a phase I/II clinical trial.
Work conducted by: Linda, Tom, Ranjan, Judit
Other ongoing projects explore the use of natural adjuvants found in common prophylactic vaccines, and an oncolytic virus (Newcastle Disease virus, in collaboration with the Garcia-Sastre lab) to induce immunogenic tumor cell death and promote dendritic cell-mediated tumor antigen cross-priming. These studies will help us to better understand how these unique adjuvants help to improve immune responses against hematopoietic cancers.
Work conducted by: Mark, Judit, Gvantsa, Kristy, Gabrielle
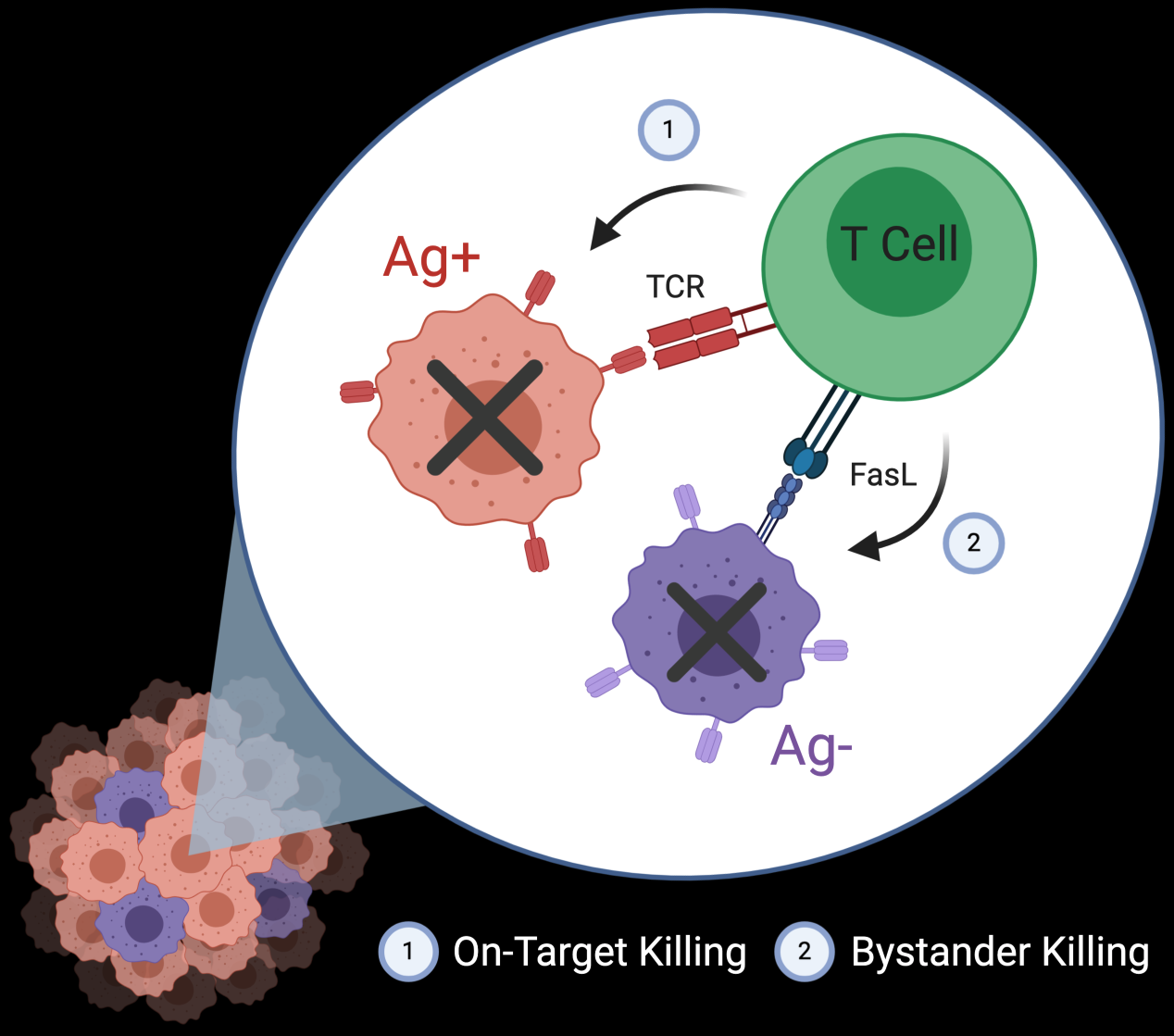
Targeted therapies lead to selective antigen loss and treatment resistance, as seen by relapse after checkpoint blockade, CAR-T, mAb therapy, and pre-defined antigen vaccines (gp100, NY-ESO-1, etc.). A method to prevent relapse is to clear Ag-negative cells. We demonstrate that off-target T cell killing (bystander killing) is mediated by FasL-Fas interactions and can be potentiated in vitro and in mouse models (Cancer Discovery, 2021). Further characterization of the geographic scope, activation status of effector cells, and molecular pathways that contribute to regional killing will help translate this finding into potential therapy.
Work conducted by: Matt, Ranjan, Gvantsa
T cells (cyan) can kill both green Ag(+) and blue Ag(-) cells; red influx indicating death; white arrow highlights bystander killing.
Cross-priming of CD8+ T cells by conventional Type 1 dendritic cells (cDC1) is necessary for effective antitumor therapy, yet there is no established method for measuring T cell cross- or direct-priming in vivo. This project seeks to define a signature of cross-primed T cells using RNA-seq of tumor-specific T cells isolated from H2Kd-/- tumor-bearing mice that can only cross-prime T cells, or allogeneic mice that can only directly prime T cells via direct tumoral antigen presentation. This will produce cross- and direct-primed T cell signatures that can be used to guide design of immunotherapies by targeting novel checkpoints or costimulatory receptors, to monitor responses to immunotherapy, and to predict responses to checkpoint blockade and similar therapies.
Work conducted by: Gabrielle, Isabella
Bruton’s Tyrosine Kinase inhibitors (BTKi) are shown to stop the growth of lymphomas and leukemias by blocking pro-survival signals in developing B cells. Evidence suggests that Ibrutinib increases engraftment and in vivo activity in mice; however, it also targets ITK, a kinase implicated in T cell proliferation and differentation. We are investigating whether treatment with Ibrutinib versus second generation Acalabrutinib affects T cells .
Work conducted by: Gvantsa, Judit, Sidorela, Moah, Linda, Jonathan
Cancer cells can also evolve anti-lymphoma properties resulting from primary resistance or immunoediting. One novel molecule we’ve identified is a transmembrane protein that is expressed and dampens anti-tumor T cell response. We are trying to understand more about this molecule and whether it can be therapeutically targeted to improve anti-lymphoma T cell therapy.
Work conducted by: Moah, Kristy, Judit, Ranjan
Homeostatic activating and inhibitory signals contribute to T cell anti-tumor activity in recipient mice and human patients. By giving checkpoint inhibitors and BMT (“immunotransplant”), homeostatic inhibition is uncoupled from activation and improves the efficacy of T cell therapy (Cancer Discovery, 2019). These findings are being actively pursued in clinical trial (NCT03305445).
Work conducted by: Netonia, Mark, Linda, Ranjan, Judit